On May 29, at the 2025 SMM (2nd) Rare Earth Industry Forum hosted by SMM Information & Technology Co., Ltd. (SMM), Liang Xingfang, the Deputy Director of the Shandong Rare Earth Research Institute, discussed the "Application and Industrial Production of Rare Earth Fluorides."
Part 1: Characteristics and Main Applications of Rare Earth Fluorides
Structural Characteristics of Rare Earth Elements
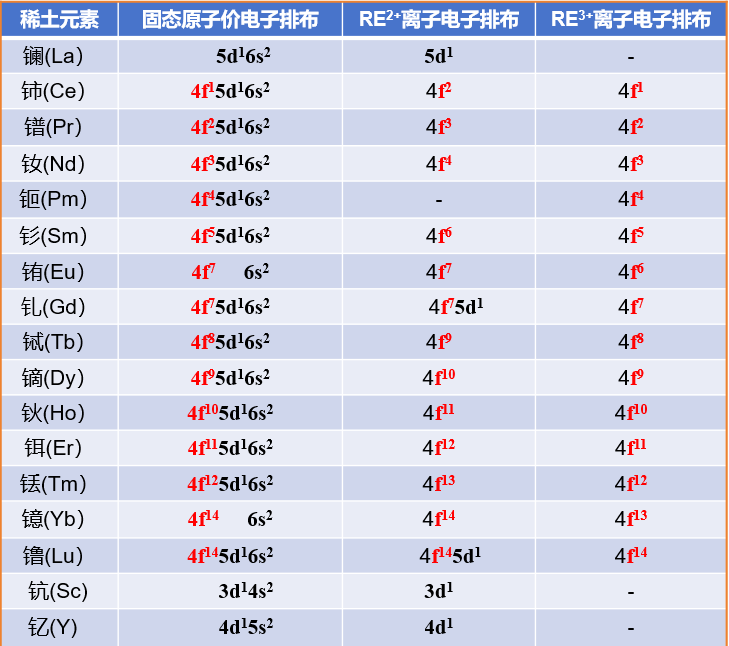
1. Scandium, yttrium, and the lanthanides share the same outermost electron configuration, resulting in similar chemical properties.
2. The number of electrons in the 4f orbital ranges from 0 to 14 from lanthanum to lutetium.
3. The 4f orbital is shielded by outer electrons, leading to energy transition differences and structural variations during electron loss, resulting in distinct properties.
4. Fluorine is one of the elements with the strongest oxidizing power.
Rare earth fluorides exhibit strong ionic bond stability.
Rare earth fluorides are compounds containing rare earth elements and fluorine.
1. Binary rare earth fluorides: Direct combination of rare earth elements with fluorine;
2. Complex rare earth fluorides: Doped with other elements to possess specific functions;
3. Nanoscale rare earth fluorides: Exhibit special size and surface effects.
Binary rare earth fluorides
High melting point; low vapor pressure; chemically stable and insoluble in water; outstanding optical properties, capable of reducing excited state energy loss and significantly enhancing upconversion luminescence efficiency.
Rare earth fluorides are important materials with unique magnetic, optical, and electrical properties.
1. Optical materials: Laser crystal materials, photofunctional materials, fluorescent materials, and optical fiber materials;
2. Catalytic materials: Catalysts for petroleum cracking and environmental protection;
3. Additives for polishing materials, ceramic materials, lubricants, and anticorrosive materials;
4. Raw and auxiliary materials for preparing rare earth metals or alloys.
Raw and auxiliary materials for producing rare earth metals and alloys via molten salt electrolysis and calciothermic reduction processes.
The applications in high-end industries and the demand from traditional industries have promoted the development of the rare earth fluoride industry.
He also elaborated on the characteristics and main applications of binary rare earth fluorides.
Main Applications of Rare Earth Fluorides in the Rare Earth Industry
1. Raw and auxiliary materials for producing rare earth metals and alloys
Auxiliary materials for molten salt electrolysis process: Electrolyte REF3+LiF
Raw materials for calciothermic reduction process: REF3+Ca
China's NdFeB production reached approximately 300,000 mt in 2024.
To produce raw materials such as Pr-Nd alloy, Nd, Ce, Gd-Fe alloy, and Dy-Fe alloy, over 5,000 mt of rare earth fluorides are required.
2. The raw materials for grain boundary diffusion of NdFeB permanent magnet materials, dysprosium fluoride and terbium fluoride, are mainly restricted by Japanese patents.
Part II: Industrial Production Technology of Rare Earth Fluorides

It elaborates on 1. the wet process (which has been listed as an obsolete process by the Ministry of Industry and Information Technology (MIIT)), and 2. NH₄HF₂ fluorination, etc.
Experience sharing on the preparation of terbium fluoride:
• Mix NH₄HF₂ with terbium oxide evenly and place in a high-purity graphite crucible
• Heat slowly from 0-150℃, hold at 150℃, and remove ammonia at 300℃-400℃ (can be less than 100ppm)
• After NH₄HF₂ fluorination, fluorinate again at 600℃ with (argon + hydrogen fluoride) gas
• Prepare terbium metal by the calcium thermal reduction method, with an oxygen content of 230ppm
Suitable for preparing rare earth fluorides of high-value elements such as scandium and terbium
3. Fluorination with hydrogen fluoride gas

Key points of process control:
Thickness of rare earth oxide furnace charge; channel for hydrogen fluoride gas; coordination between the temperature rise regime and the hydrogen fluoride gas flow rate; the biggest issue is the poor contact between the solid and gas in the solid-gas reaction; how to keep the rare earth oxide material in motion to increase its contact surface with the hydrogen fluoride gas.
Fluidization and rolling.
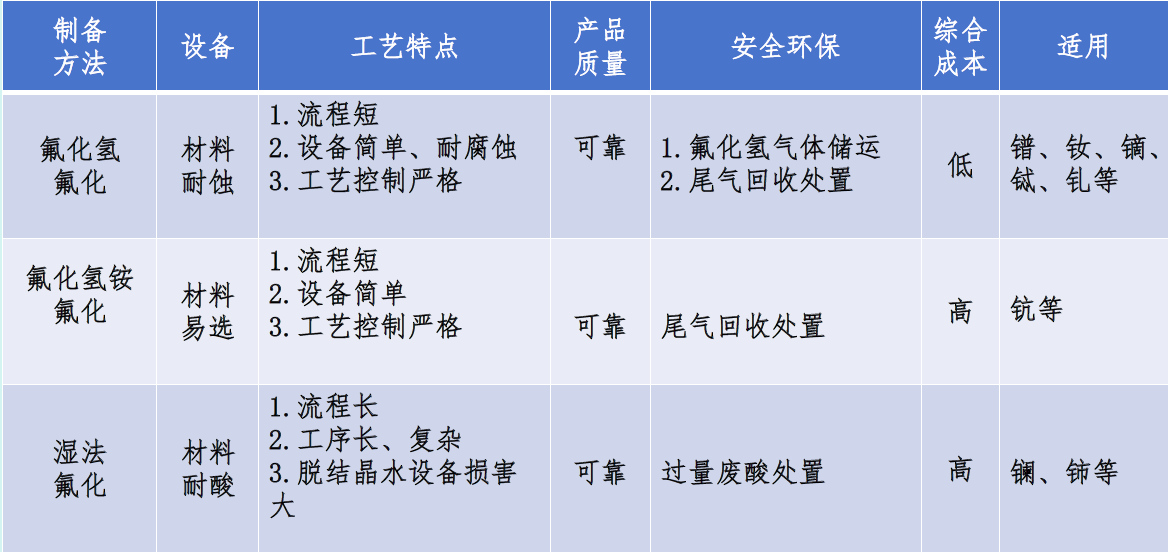
Comparison of industrial production technologies for rare earth fluorides
Part III: Problems and Directions of Current Rare Earth Fluoride Production Methods
◇ The hydrogen fluoride method, NH₄HF₂ method, and wet process each have their own advantages, depending on the product characteristics.
◇ The production equipment for dry fluorides is not entirely reasonable, and the level of intelligence is not high; there is a caking phenomenon, incomplete solid-gas reaction, and the application of fluidized beds; process control for material thickness, gas feed rate, reaction temperature, and water vapor drainage.
◇ Corrosion resistance of lining materials
◇ Rare earth fluorides are prone to forming oxyfluorides at around 780℃
They are reduced to rare earth fluorides under a hydrogen fluoride atmosphere at 1700℃.
◇ After adopting the wet dehydration process + NH₄HF₂ (or hydrogen fluoride gas method)
NH₄HF₂ fluorination + hydrogen fluoride gas fluorination
Rare earth fluorides with lower oxygen content can be obtained
◇ High chemical purity and outstanding physical properties
High purity (4N), ultra-high purity (greater than 6N)
◇ Nanomaterials, composite rare earth fluoride materials with comprehensive functions.



